
Ensuring access to the European market requires a comprehensive understanding of the National and Union level chemical Regulations and Directives. As legislators introduce further regulations and enforcement measures for non-compliance in their continued efforts to protect human and environmental health, organisations can minimise future risk and ensure customer satisfaction by maintaining compliance.
Failure to abide by regulations can result in enforcement actions including goods being stopped at import, fines, and criminal prosecution.
Working with a reliable, informed partner can ensure organisations have maximum notice of the impending changes affecting their products, while providing insight to the future regulatory landscape.
CLP | Poison Centres | REACH | Safety Data Sheets | Horizon Scanning | Solutions for your business
Considerations for European compliance
Classification, Labelling and Packaging Regulation - CLP
The Classification, Labelling and Packaging (CLP) Regulation applicable in the EU aligns with the United Nations’ Globally Harmonised System (GHS) of Classification and Labelling of Chemicals, with the aim of ensuring a high level of protection for health and the environment. CLP requires manufacturers, importers, and downstream users of substances, mixtures, or articles to classify, label and package their chemicals appropriately before placing them on the market.
Under Article 45 of CLP, entities placing mixtures classified for health or physical hazards on the market within the EU must provide information for health-related emergencies to nationally appointed bodies via portal operated by the European Chemicals Agency (ECHA). National law can also mean each country within the EU may have additional requirements.
Due to the dynamic nature of the chemical landscape, changes to the CLP regulation are frequent. “Adaptations to Technical Progress” (ATPs) to the CLP regulation are published at least annually, and may contain changes such as new harmonised classifications, or new labelling requirements. These changes may have an impact on Safety Data Sheets (SDS), labels, REACH registration dossiers, and obligations derived from other regulations. It is important that suppliers remain aware of the changes to the classification status of their substances and mixtures, and of those used in their products.
Stay ahead of risks: maintain compliance with our horizon scanning tool
Poison centres
Poison centres play an important role in the safe use of chemicals and provide both protective and remedial measures in case of poisoning incidents. They provide medical advice to members of the public and healthcare professionals during exposure incidents relating to hazardous products.
Poison centres in the Member States of the European Union answer over half a million calls for support each year. Roughly half of these are related to accidental exposures involving children – the information provided to Poison Centres has a direct impact on human lives.
Ensuring your hazardous products are appropriately notified and updated is a crucial part of maintaining your market access, protecting your reputation with customers and key stakeholders within each Member State, and streamlining the movement of your goods.
Although the harmonised submission of information in the Annex VIII format through the ECHA portal has simplified notification for many, there are still several EU Member States that have additional requirements, including fees and other reporting obligations.
Understanding the specific requirements for each Member State your products enter is essential, as this level of detailed information is not currently reported by ECHA.
Working closely with each Member State poison centre since the introduction of the legislation has enabled Ricardo to establish robust relationships and immediate notification of changes to- or additional requirements.
EU REACH
The European Union’s Registration, Evaluation, Authorisation, and Restriction of Chemicals (EU REACH) Regulation aims to improve the protection of human health and the environment from the risks that can be posed by chemicals, while enhancing the competitiveness of the EU chemicals industry.
EU REACH Registrations
EU REACH requires companies to register the substances they manufacture or import in the EU above 1 ton per year and identify and manage the risks associated. If risks cannot be managed safely, authorities may take more stringent measures such as restrictions or bans on the use of the substance.
Ricardo offers a full suite of chemical regulatory solutions that can be tailored to your business and supply chain.
As REACH registrations must be held by a company based physically within the EU or UK (for EU REACH and UK REACH respectively), Ricardo also offers an Only Representative (OR) service to manufacturers and formulators based outside of these areas. By shifting the regulatory burden away, Ricardo’s clients find that their products become more attractive to UK and EU based customers who can simply order the product and be assured of regulatory compliance and market quality.
By using Ricardo’s REACH team our clients can plan for market launches, receive advice on label requirements which helps anticipate market reception, and give their customers peace of mind that all regulatory compliance is addressed before they order the product.
Our solution can also include authoring of extended SDS (e-SDS), required for compliance with the EU REACH Regulation where manufacture or import exceed 10 tonnes per year.
Safety Data Sheets
Safety Data Sheets (SDS) provide vital information relating to the potential hazards and physiochemical properties of a product to enable safe use, as well as emergency response, transport, and other regulatory information.
Failure to provide sufficient information on an SDS can cause delays in accessing markets, impact supply chains, increase costs, and increase the risk of harm to people, assets, reputation and the environment.
Companies may also lose business from poor quality SDS. Customers receiving non-compliant SDSs may require corrections before progressing with a purchase, and audits may identify inadequate SDSs for action. Customers are likely to opt for companies presenting thorough and up-to-date documentation first time, demonstrating attention to detail and ability to react to regulatory changes.
Compliant SDSs will not only be safer, but also more economically feasible as they reduce the need for corrective actions thereby increasing confidence in those using the SDS.
Safety Data Sheet – the core of compliance
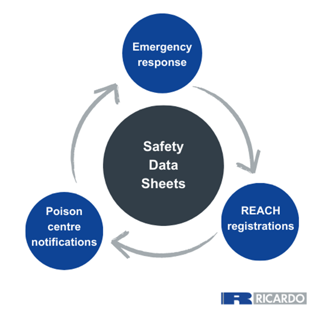
Providing high-quality SDS is essential to maintain regulatory compliance for REACH, Poison Centre Notifications and providing adequate support for emergency response situations. Acting as an essential data point in all these regulatory areas, having compliant and fit-for-purpose SDS is vital for market access into Europe.
Our dedicated in-house team have many years of experience in compiling, reviewing, translating, and submitting SDS on behalf of industry leading chemical organisations. Our end-to-end solution enables our customers to achieve compliance across all territories with no subscription required.
Horizon Scanning – Future proofing compliance
Ricardo’s horizon scanning and compliance reporting tool offers a substance inventory check against global regulatory lists, and provides the results in a clear, easy-to-read report enabling organisations to quickly determine the status of their substances.
The risk management process for substances is complex. Understanding the hazards of chemicals is a key part of responsible product stewardship – essential for a sustainable business and for maintaining compliance with global chemical regulations. The European Chemicals Agency (ECHA) updates its lists, including Authorisation, Candidate, CoRAP, PACT and Restriction, frequently and often resulting in the movement of substances between lists. Each list carries its own regulatory implications and mitigation steps. The appearance or movement of a substance within these lists can trigger actions for organisations, such as phasing out a certain substance from product range, altering its concentration, or introducing specific workers’ safety procedures.
Monitoring these various REACH lists can be time consuming and problematic because of the introduction of generic group entries, which means it is no longer enough to simply search for numeric chemical identifiers.
Ricardo’s horizon scanning tool identifies the information pertaining to the product portfolio and sends notifications, minimising the likelihood that changes requiring action are missed, as these could result in non-compliance. This protects reputations and safeguards against enforcement actions, supporting organisations to future-proof their product portfolio.
Learn more about our horizon scanning tool
Solutions for your organisation
Ricardo’s expertise in the field of European chemical regulations ensures compliance as well as providing insight into the ever-changing regulatory landscape. Our chemical and sustainability experts support chemical manufacturers and distributors around the world, helping to reduce risk, deliver operational best practice and improve the long-term sustainability of operations, products, and services.
Tailoring our services to the needs of your organisation, we ensure compliance with EU legislation is maintained at all times and provide pro-active notifications of changes that may impact your organisation.





 Follow Ricardo Chemical Solutions for regular updates
Follow Ricardo Chemical Solutions for regular updates




